The Importance of ISO 9001 and ISO 13485 Certifications in Electronics Manufacturing

For electronics manufacturers striving for excellence, ISO 9001 and ISO 13485 certifications serve as the foundation for quality, reliability, and compliance in a rapidly evolving industry. These certifications help manufacturers demonstrate their commitment to maintaining high-quality standards and adhering to global regulations.
For electronics manufacturers involved in industrial automation, telecommunications, automotive, aerospace, and medical device production, these certifications are more than just industry best practices—they are essential credentials that differentiate reliable manufacturers from those who may lack the necessary quality control measures.
Whether an electronics manufacturer is producing industrial circuit boards, medical imaging devices, or high-reliability telecommunications components, obtaining ISO 9001 and ISO 13485 certifications ensures that their processes meet global quality benchmarks.
ISO 9001: The General Quality Management Standard
What is ISO 9001?
ISO 9001 is an internationally recognized quality management system (QMS) standard applicable to organizations across various industries, including electronics manufacturing. It provides a structured framework for companies to establish, maintain, and continuously improve their quality management systems, ensuring customer satisfaction, regulatory compliance, and operational efficiency.
This standard covers several key aspects of quality management, including:
- Context of the Organization: Companies must assess both external and internal factors that influence their ability to meet quality objectives and maintain efficiency.
- Leadership Commitment: Strong leadership is essential in implementing and sustaining an effective QMS. ISO 9001 emphasizes management’s role in driving quality culture.
- Strategic Planning: Organizations must establish measurable quality objectives and develop plans to achieve them, ensuring continuous improvement.
- Operational Control: Companies must implement structured processes to meet customer requirements, ensuring consistency, reliability, and high product quality.
- Performance Monitoring: Regular audits and performance evaluations are necessary to identify areas for improvement and optimize processes.
- Resource & Support Management: Proper allocation of resources, employee training, and effective communication are critical to maintaining a high-functioning QMS.
Why ISO 9001 Matters for Electronics Manufacturing
Electronics manufacturers benefit significantly from ISO 9001 certification as it helps establish robust quality control systems, reducing errors and ensuring consistency across production. Here’s why it is essential:
- Consistent Product Quality: Implementing standardized processes reduces defects and ensures uniformity in manufacturing.
- Improved Customer Confidence: Certification reassures customers that a manufacturer follows strict quality control protocols.
- Operational Efficiency: Streamlining workflows enhances productivity, reduces costs, and minimizes waste in production.
- Regulatory Compliance: Many industries, including telecommunications, industrial automation, and aerospace, require ISO 9001 certification from suppliers.
- Data-Driven Improvement: Performance monitoring ensures companies use real-time data to enhance operations and adapt to industry changes.
- Stronger Market Position: ISO 9001-certified manufacturers stand out in the market and are often preferred by customers seeking reliable electronics solutions.
For electronics manufacturers, ISO 9001 serves as the foundation for quality assurance, risk management, and long-term operational success.
ISO 13485: The Medical Device Manufacturing Standard
What is ISO 13485?
ISO 13485 is a quality management standard specifically designed for medical device manufacturers, including those producing medical-grade electronics. While it builds upon ISO 9001, it incorporates stricter regulatory requirements focused on patient safety, risk management, and compliance with international medical device regulations.
Key aspects of ISO 13485 include:
- Customer and Patient Safety: Requires rigorous post-market surveillance, including monitoring customer complaints, product defects, and regulatory changes.
- Regulatory Compliance: Ensures adherence to medical industry requirements, including FDA (U.S.), EU MDR (Europe), and Health Canada regulations.
- Risk Management: Establishes risk-based decision-making throughout the product lifecycle, preventing failures that could impact patient safety.
- Product Traceability: Requires comprehensive documentation and tracking throughout the supply chain, essential for audits and recalls.
- Process Validation: Ensures that production, sterilization, and testing processes meet medical device standards for safety and reliability.
Why ISO 13485 Matters for Electronics Manufacturers
For electronics manufacturers producing medical devices, ISO 13485 certification is not just an advantage—it’s a requirement. Here’s why:
- Regulatory Readiness: Compliance with global regulations prevents legal risks and facilitates market access.
- Higher Safety Standards: Strict risk management protocols reduce the likelihood of product failures.
- Competitive Advantage: Certification enables manufacturers to enter the highly regulated medical electronics sector.
- Stronger Quality Control: Enforces more rigorous testing, validation, and compliance processes.
- Customer Trust: Medical device companies prefer working with ISO 13485-certified electronics manufacturers to ensure product reliability.
For electronics manufacturers aiming to expand into medical device production, ISO 13485 is a non-negotiable standard that ensures compliance, patient safety, and high-quality manufacturing.
The Importance of These Certifications for Electronics Manufacturers
For an electronics contract manufacturer like August Electronics, achieving ISO 9001 and ISO 13485 certifications reflects a deep commitment to quality, reliability, and regulatory compliance.
Industries such as industrial automation, medical technology, automotive, telecommunications, and aerospace rely on certified manufacturing partners to ensure product safety, durability, and compliance with strict regulations.
Key Benefits for Electronics Manufacturers:
- Enhanced Brand Reputation: Certification demonstrates credibility and a commitment to excellence, improving customer trust.
- Regulatory Compliance: Ensures that products meet international standards, reducing the risk of legal or regulatory complications.
- Expanded Market Access: Many businesses and government contracts require manufacturers to hold ISO 9001 and ISO 13485 certifications before entering into agreements.
- Better Quality Assurance: Enables a structured approach to defect prevention, testing, and validation, ensuring high-quality electronic components.
- Increased Customer Retention: Clients prefer to work with certified manufacturers, knowing that their electronic components meet stringent quality and compliance requirements.
- Improved Process Control: Standardized procedures help manufacturers optimize workflows, increase efficiency, and minimize production waste.
For many electronics manufacturers, ISO certifications are not just about compliance—they are a strategic investment in long-term business success.
How to Obtain ISO 9001 and ISO 13485 Certifications
Achieving ISO 9001 and ISO 13485 certifications requires manufacturers to undergo a rigorous evaluation process. Here’s an overview of the steps involved:
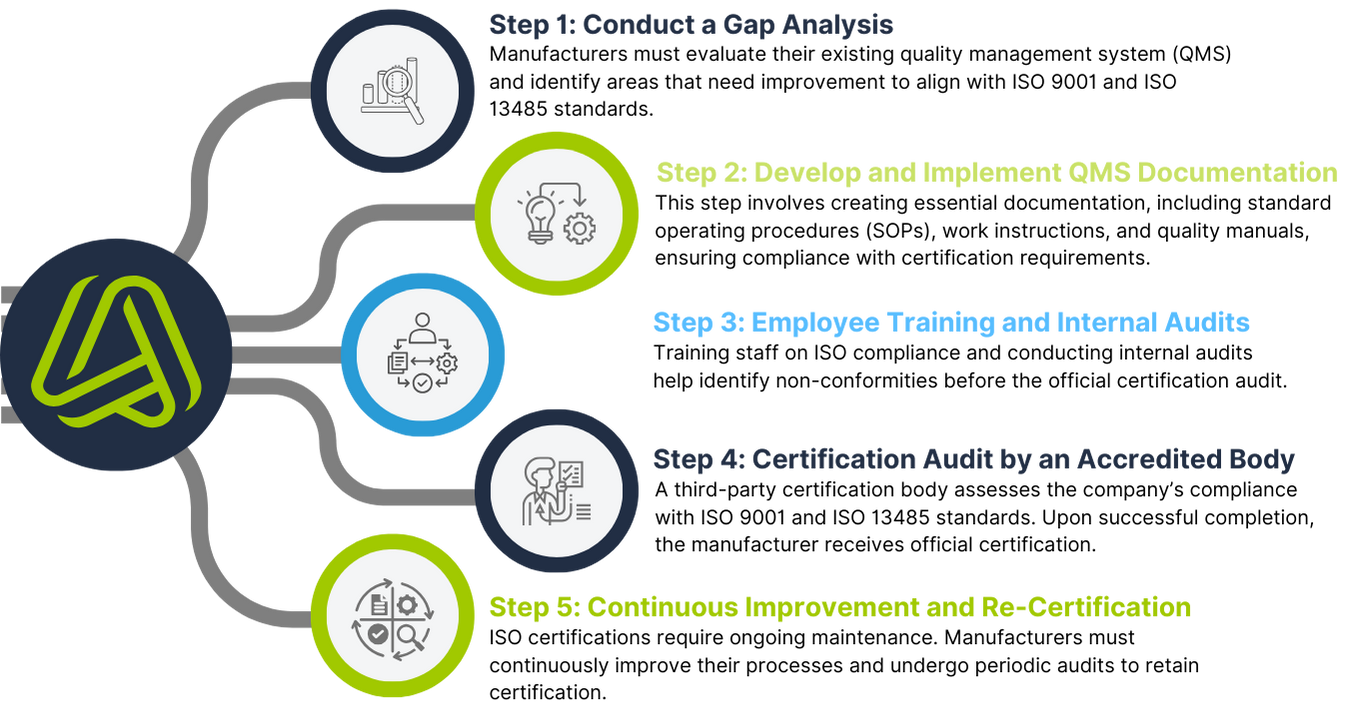
Final Thoughts
As the electronics manufacturing industry continues to become increasingly competitive and regulated, ISO 9001 and ISO 13485 certifications are critical for ensuring product quality, compliance, and market success. These certifications help manufacturers establish credibility, meet industry regulations, and expand into new markets, particularly in industrial automation, telecommunications, and medical technology.
For electronics manufacturers seeking long-term growth, enhanced customer trust, and a competitive edge, obtaining ISO 9001 and ISO 13485 certifications is an essential step toward achieving operational excellence and sustainable business success.
At August Electronics, we are proud to be ISO 9001 and ISO 13485 certified, ensuring that every product we manufacture meets the highest standards of quality and compliance. Whether you need a trusted partner for industrial, automotive, medical, or high-reliability electronics manufacturing, our team is ready to help.
Partner with August Electronics
Looking for a reliable ISO-certified electronics manufacturer? Contact August Electronics to discuss how we can support your next project with precision, efficiency, and industry-leading quality.
